Neutron
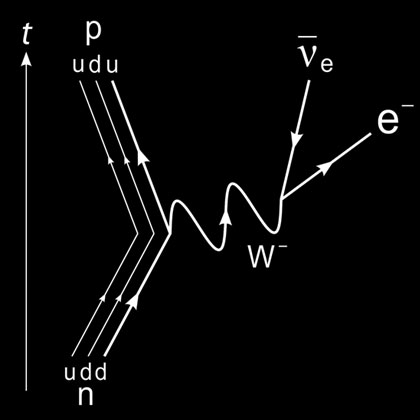
Image: The Feynman diagram for the beta-decay of a neutron into a proton. The down quark in the neutron decays into an up quark to make a proton, emitting an electron and an electron anti-neutrino.
Neutrons are neutral particles comprising bound states of three quarks, of mass
1.67 x 10-27, slightly greater mass than the proton. It has half-integral spin and is a baryon, subject to the strong and weak nuclear forces. In isolation a neutron will decay to a proton, electron and anti-neutrino by β-decay, with a half-life of about 8 minutes (881.5 sec.). When bound in roughly equal numbers with protons they give rise to stable nuclei that make up ordinary matter. Atoms consist of stable nuclei in bound states with electrons. The chemical elements correspond to atoms of differing proton numbers: their chemical properties (apart from density) only depend on the nucleus through its electrical charge, hence the proton number. Variation of neutron number for fixed proton number yields distinct isotopes of the element, of varying stability.
The binding energy per nucleon increases with proton number for lighter elements, but decreases for heavier elements, with a peak at Iron, Fe56. Thus energy can be extracted from nuclei by fusion (for light elements) but by fission (for heavy elements). Fission, particularly for the trans-uranic elements, can happen spontaneously, but for fusion to take place it is invariably necessary to overcome the electrostatic repulsion of the nuclei involved. In all circumstances this requires immense temperatures and pressures.
The neutron was first conjectured by Rutherford in 1920. It was first observed by Bothe and Becker in 1931, as energetic rays emitted by light atoms bombarded by alpha particles (energetic helium neuclei); however, they thought them gamma-rays. In 1932 James Chadwick showed they were rapidly absorbed in nitrogen and other gases, and was able to estimate their mass. For this he was awarded the Nobel Prize for physics in 1935.
Links
Wikipedia, Chronology of the Universe >

